Research
-

Research Interests- Dengue virus immunity and pathogenesis
Dengue is an acute febrile illness caused by any of four related flaviviruses (dengue virus [DENV] serotypes 1-4), small enveloped viruses containing a non-segmented positive-sense RNA genome. DENV infection is acquired through a transmission cycle between humans and mosquitoes of the genus Aedes, principally A. aegypti.
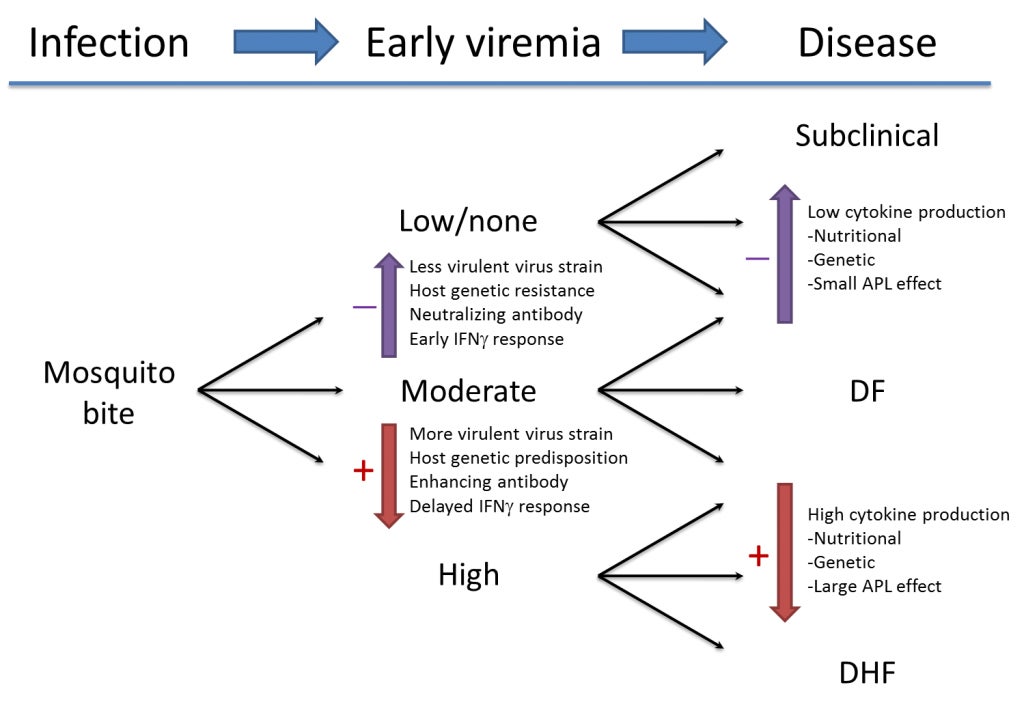
In humans, there is a wide spectrum of clinical manifestations of DENV infection. This ranges from an uncomplicated and self-limited febrile illness (dengue fever, DF) to a plasma leakage syndrome accompanied by bleeding (dengue hemorrhagic fever, DHF). Although DHF is observed in only a small percentage of DENV infection, it plays a large role in the public health problem of dengue because it can lead to shock and death.
Many factors contribute to the risk for DHF, but one of the most important factors is pre-existing immunity from an earlier DENV infection. Infection with one DENV only provides long-lasting protection against that serotype; sequential infection with multiple different DENV serotypes is therefore possible, and, because of the increasing global circulation of DENV, this has been occurring with increasing frequency in tropical areas of the world. The basis for the association of DHF with secondary DENV infection is a major subject of research in Dr. Rothman’s laboratory. We hypothesize that both the quality and timing of DENV-specific antibody and T lymphocyte responses influence whether their overall effect is beneficial (controlling DENV replication and reducing the severity of illness) or harmful (creating a ‘cytokine storm’ leading to plasma leakage). The results of our research will be applied to evaluation and management of patients with dengue and to development and testing of vaccines and therapeutics against dengue.
 Home
Home Browse
Browse Close
Close Events
Events Maps
Maps Email
Email Brightspace
Brightspace eCampus
eCampus


